Landscape architects have a growing responsibility to contribute to the revival of biodiversity and ecosystem services. Increasingly we are also asked to design landscapes that are more resilient to rapid environmental change and unpredictable extreme events. This requires us to incorporate ecological principles into our work. One strategy is using ecologically driven planting design that strives to enhance biodiversity at the genetic, species, community, and ecosystem levels [1]. While many practitioners understand the importance of increasing species and habitat diversity, we feel that there is still a lack of focus on the benefits of incorporating diversity below – and above- the species level into planting designs. For the long-term ability of urban and landscape designs to persist, with resistance and resilience to climate chaos, old and new diseases and pests, and the capacity to continue to evolve, this kind of multi-layered, or nested hierarchy of levels of diversity in plant assemblages is essential [3]. This is true in both natural and constructed environments.
We carried out a study with Mathews Nielsen Landscape Architects (MNLA) to understand how to incorporate more genetic or within-species diversity into the planting design of Pier 42, a waterfront redevelopment project in Lower Manhattan. In this essay, we make the following contributions: 1) explain what genetic, or within-species diversity is; 2) discuss how this level of biodiversity might benefit urban green space design; 3) review how horticultural propagation regimes typically reduce genetic diversity; and 4) introduce two strategies that can be used to overcome this latter issue.
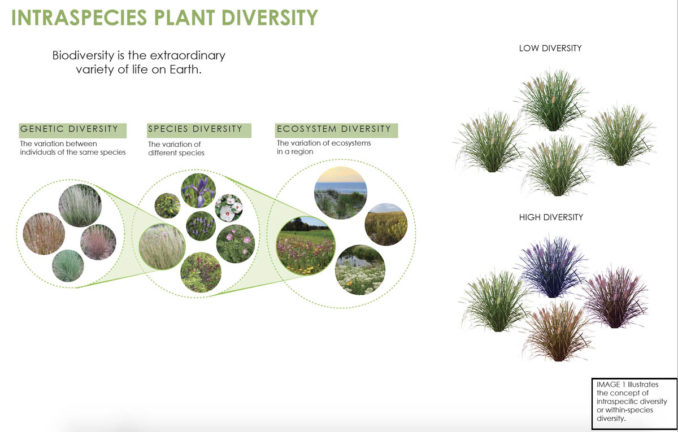
What is genetic diversity, and why is it important?
Genetic diversity is one of the essential and foundational “building blocks” of biodiversity [4]. Biodiversity – another word for the extraordinary variety of life on Earth in its forms and interactions -can be organized for the sake of study into interdependent levels. Genetic diversity forms the base of the biodiversity hierarchy; next is individual species, then communities, and finally, entire ecosystems (Image 1) [5]. As Beck (2013) points out, in nature, if we look closely, we can see within-species as well as inter-specific diversity. Dozens to thousands of individuals comprise plant populations. Each plant can exhibit slightly different traits from the others, including germination rates, size, age, the timing of flowering and setting seeds, tolerance to heat, cold, and extreme weather events, resistance to disease and pests, etc. [6]. This variation is known as intraspecific or within-species diversity. In simple terms, genetic and environmental factors cause the bulk of the variability that exists among individuals of the same species. However, if that variation is heritable, then it has a genetic basis and serves as the raw material on which natural selection operates to solve problems [7]. Here we define genetic diversity as any measure that quantifies the magnitude of genetic variability among individuals of the same species. Genetic diversity can influence how a members or populations of a species will interact with other species and function within an ecosystem [8-9]. For resilience, it is crucial that genetic diversity is maintained as it provides the basis for plant populations to respond and adapt to environmental and climatic shifts and uncertainty [10].
How might genetic diversity benefit landscape design?
A growing body of research indicates that genetic diversity in plant populations is a prerequisite for long-term evolutionary change and ecosystem functioning [11, 12]. Several compelling examples show how genetic diversity helps to underpin plant populations’ capacity to establish rapidly, resist invasion, recover from episodic herbivory and extreme weather events, and adapt to climate change [13, 14, 15]. For example, a study by Williams (2001) on a species of eelgrass (Zostera marina), an essential species in estuarine ecosystems and the restoration thereof, demonstrated that reduced genetic variation in the plant material used in restoration resulted in decreased population growth and individual fitness [16]. In another study using the same widespread species of eelgrass, Reynolds et al. (2012) found that increasing the genetic diversity of planting material used in the restoration seagrass ‘meadows’ of this species resulted in plants that grew in density more quickly, survived longer, and provided more ecosystem services such as invertebrate habitat, increased primary productivity, and nutrient retention than plantations with narrower genetic diversity [17]. Thus, genetic diversity can have favorable ecological consequences at the population, community, and ecosystem levels. In some cases, the effects are comparable in magnitude to the impact of species diversity [18].
How is genetic diversity lost through horticultural propagation?
Horticultural propagation regimes typically subject plant populations to genetic sampling, selection for certain traits, and artificial selection to favor those traits. The primary way that genetic diversity is lost is through seed collection. Collectors might harvest seed from a small portion of a large population or from a subset of plants that flower at a specific time. This can produce a genetic bottleneck. From then on, practices like seed cleaning, storage, and stratification, seed sowing, seedling management, packaging, and transportation can continue to influence the genetic diversity of planting stock [19].
For example, seed handlers may inadvertently lose genetic diversity if they discard lighter and smaller seeds during the cleaning process. Nursery growing conditions also expose plants to novel environments. Stress is not as severe and heterogenous as in nature because growers can control factors like light, temperature, fertilization, irrigation, pests, and diseases. Nursery conditions inevitably impose artificial selection on plants, affecting genetic diversity and adaptation within a few generations. In other cases, the loss of diversity may be more intentional. For example, growers might select larger, more uniform, or faster-germinating plants and cull the others that do not meet these criteria [20].
Plant breeders also deliberately reduce variability to develop cultivars with uniform traits. For example, consider the cold-hardy perennial hibiscus species Hibiscus moscheutos. For example, they have produced a vast and dazzling array of cultivars like H. moscheutos ‘Luna Red,’ ‘Disco Belle Pink,’ ‘Disco Belle Rosy Red,’ ‘Disco Belle White,’ ‘Luna Pink Swirl,’ ‘Who says,’ and ‘Wildwood Wonder,’ among many others. Once a crossing creates a combination of preferred dominant genes, further offspring are reproduced asexually through divisions, cuttings, or micropropagation, rendering each plant genetically identical to the original hybrid parent plant.
How can we incorporate genetic diversity into planting designs?
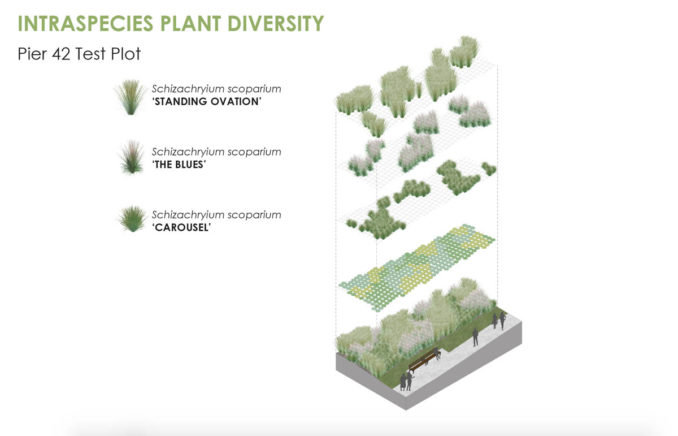
During the design phase of MNLA’s Pier 42 project, we investigated feasible methods to increase the within-species diversity of the proposed planting list. Pier 42 is a site along Lower Manhattan’s East River shoreline that the city is transforming into a public park. Our study did not include carrying out genetic analyses. Instead, we gathered variety, cultivar, and breeding information for twenty-two of the species in the proposed planting list (i.e., grasses and perennials). We did this by consulting various internet databases (e.g., Tropicos®, of the Missouri Botanical Garden, Lady Bird Johnson Wildflower Center), peer-reviewed articles, and wholesale nursery catalogs. With this baseline information, we generated strategies to increase the intraspecific diversity of each species. The results of our inquiry yielded two main approaches: a mixed ecotype and multiple varieties or cultivar approach.
The mixed local ecotype approach
We found that the optimal way to incorporate genetic diversity into a planting design by proxy is to source planting material that contains a mix of local ecotypes. Plant species can occupy a wide range of habitat conditions through adaptions driven by natural selection. Over time, local adaptation to a particular climate or microclimates can lead to the emergence of a variety or a population within a single species that is genetically distinct [21]. Therefore, ensuring that planting material contains a mix of ecotypic differences is a potentially sound method to enhance the genetic diversity in an urban landscape design project.
Our study found that it was possible to use the local ecotype approach for any species that have natural populations growing in proximity to the project site (e.g., 50-150 miles). The genetics of local plant populations represent thousands of years of adaption to a particular area. Therefore, landscape architects should use locally sourced plant material when it is available to maintain local biodiversity. However, we recognize that local sources of plant material might not always be available or accessible. Furthermore, some projects may require collecting seed and organizing contract growing to use locally sourced material, which may not be feasible because of time and budget constraints.
We found that it would have been feasible for our project to follow a local ecotype approach for several of the proposed species. However, because this is a public project, we understood that it would be challenging for the New York City Department of Parks & Recreation (NYC DPR) to require their contractors to source planting material from specific locations. We did annotate the planting schedule to suggest that planting material for one species, Iris prismatica, should be sourced within a certain distance from the project site (e.g., 50 or 100 miles). We highlighted its threatened status as further justification for procuring plant material with local ecotypes.
The multiple varieties or cultivar approach
We found that a complementary method to incorporate within-species diversity into an urban planting design is to increase the number of varieties or cultivars of any one species. In our study, it was not feasible to follow the local ecotype approach for every proposed species. Either a species was not native to the NYC area, or plant material containing local ecotypes was not available at commercial nurseries.
The multiple varieties or cultivar approach gleans inspiration from the centuries-old cropping technique that is still widely practiced in farmers’ fields over the globe [22]. Although variety and cultivar are terms sometimes used interchangeably, there are differences between the two. Most cultivars are developed and maintained by plant breeders and horticulturists. A variety is a plant with a specific set of characteristics due to natural phenomena or artificial selection. Plants grown from the seeds of a variety will produce true-to-seed plants, whereas a cultivar will not. Once plant breeders develop a cultivar, it is usually clonally propagated to make more plants for market trade or agricultural use. Scientists have proven that planting fields with a mixture of varieties or cultivars of one species is an effective method for increasing resilience to biotic and abiotic perturbations [23]. Genotypically diverse variety or cultivar mixes can reduce pathogen transmission from one plant to another and influence yields. Increased levels of variety diversity can also translate into greater floral abundance and diversity of pollinators, which suggests that increasing within-species diversity may improve pollination services [24]. A study by Kaut et al. (2009) shows that variety mixing also enhances a plant population’s ability to resist invasion by other plant species [25]. We found that increasing the number of varieties or cultivars was a straightforward and uncomplicated way to increase within-species diversity. Therefore, we decided to incorporate between two to four different varieties or cultivars of each proposed species in the list.
When selecting multiple varieties or cultivars for a project, landscape architects could follow a few general guidelines. For example, it is important to choose varieties or cultivars that have originated or are known to perform well in ecoregion or hardiness zone where the project is located. Selecting varieties or cultivars that flower at different times during the season can help reduce the likelihood of cross-fertilization while benefiting pollinators. Installing the plants tightly together will also help maintain distinct varieties or cultivar types, as any seeds produced by the plants will have a more challenging time establishing with less open space.

What are the next steps?
The planting material propagated and distributed through nurseries is becoming more genetically uniform and losing potential resiliency to stresses while threats are increasing. Thus, there is a critical need to re-evaluate and improve seed collection and plant propagation practices at commercial nurseries. Many nursery catalogs describe what a plant looks like—flower color, height, and shape —but information on where plants originate or how the nursery propagated them is almost always lacking. We argue that landscape architects have an essential role in raising awareness and helping to shift industry practices.
Greening projects spanning from micro-scale (e.g., street tree plantings) to macro-scale (e.g., coastal restoration) require seeds and plant propagules. This physical living material forms the foundation of both natural and constructed landscapes. In addition, vegetation plays a critical role in providing an array of regulating, provisioning, and cultural ecosystem services that greatly benefit human communities. Recent studies indicate that genetic diversity in plant populations is crucial for long-term evolutionary change and ecosystem functioning. However, there is limited information on the outcomes of increasing within-species diversity in urban landscape design projects.
There is also the issue of connecting theory to practice. Landscape architects face numerous constraints during the design, planning, and implementation phases of projects (e.g., budget, timeframe, maintenance capacity, etc.) that challenge ecologically driven planting design. This study area merits more attention to shrink research gaps and develop feasible methods to incorporate increased levels of biodiversity into greening projects.
Conclusions
Increasingly landscape architects are being asked to design in response to the complex needs of a changing environment. Incorporating ecological thinking into the design process is no longer an option or an alternative; it is a necessity. Genetic diversity forms the base of the biodiversity hierarchy and plays a key role in evolutionary and ecological processes. We undertook research that yielded two strategies that can assist landscape architects in increasing plant biodiversity in their projects.
We hope that this essay spurs further communication among landscape architects, urban planners, plant breeders, horticulturists, restoration ecologists, conservation biologists, and policymakers. To date, this topic is understudied, and more multi-disciplinary research is required to close gaps between theory and practice. We must work together to figure out viable ways to incorporate within-species diversity into our designed landscapes to enhance resilience in the face of short and long-term changes in climate, and other global changes.
Designing for change
Allen E.1*, Bourne M.1, Nielsen S.1
Mathews Nielsen Landscape Architects, 120 Broadway, New York, NY 10271
* Eve Allen is a Master in City Planning Candidate at the Massachusetts Institute of Technology; she was affiliated with Mathews Nielsen Landscape Architects while carrying out research presented in this article.
Corresponding author email: eveallen@mit.edu
Image Credits: Allen E.1*, Bourne M.1, Nielsen S.1
Acknowledgements:
We thank James Aronson, Senior Scientist Emeritus at the Missouri Botanical Garden and Steering Committee member at the EcoHealth Network, for his contributions to this essay.
References:
1. Gadient, H., Küffer, C., & Stapfer, A. 2016. Bridging the Gap Between Landscape Architecture and Ecology in Teaching and Design Practice. In Bridging the Gap. ECLAS Conference 2016, Rapperswil, Switzerland. No. 14: 101.
2. Lohr, V. 2013. Diversity in Landscape Plantings: Broader Understanding and More Teaching Needed. Hort. Tech. 23 (1): 126-129.
3. Hughes A.R., Inouye B.D., Johnson M.T.J., Underwood N., Vellend M. 2008. Ecological consequences of genetic diversity. Ecol. Lett. 11: 609–623.
4. Frankel, O. H., Anthony H. D., Burdon, J.J. 1995. The Conservation of Plant Biodiversity. Cambridge University Press, Cambridge, England.
5. Frankel, O. H. 1974. Genetic conservation: Our evolutionary responsibility. Genetics 78:53–65.
6. Beck, T. 2013. Principles of Ecological Landscape Design. Island Press, St. Louis, MI.
7. Fisher, R. A.1930. The Genetical Theory of Natural Selection. The Genetical Theory of Natural Selection. Clarendon Press, Oxford, England.
8. Johnson, M.T.J., Stinchcombe, J.R. 2007. An emerging synthesis between community ecology and evolutionary biology. Trends Ecol. Evol. 22: 250–257.
9. Kettenring, K.M. et al. 2014. Application of genetic diversity– ecosystem function research to ecological restoration. App. Ecol. 51: 339–348.
10. Lewontin, R. C. 1974. The genetic basis of evolutionary change. Columbia University Press, New York, United States.
11. Kettenring, K.M. et al. 2014. Application of genetic diversity– ecosystem function research to ecological restoration. App. Ecol. 51: 339–348
12. Naeem, S., Bunker, D., Hector, A., Loreau, M. & Perrings, C. 2009. Biodiversity, Ecosystem Functioning, and Human Wellbeing: an Ecological and Economic Perspective. Oxford University Press, Oxford, England.
13. Reusch, T. B. H., Ehlers A., Hämmerli A., and Worm, B. 2005. Ecosystem Recovery after Climatic Extremes Enhanced by Genotypic Diversity. Proceedings of the National Academy of Sciences 102(8): 2826–2831.
14. Rice, K.J., and Emery, C. N. 2003. Managing microevolution: restoration in the face of global change. Front. Ecol. Environ. 1(9): 469-478.
15. Crutsinger, G.M., Souza, L. & Sanders, N.J. 2008. Intraspecific diversity and dominant genotypes resist plant invasions. Ecol. Lett. 11:16–23.
16. Williams, S. L. 2001. Reduced Genetic Diversity in Eelgrass Transplantations Affects Both Population Growth and Individual Fitness. Ecol. App. 11(5): 1472–1488.
17. Reynolds L.K., McGlathery K.J., Waycott M. 2012. Genetic diversity enhances restoration success by augmenting ecosystem services. PLoS ONE. 7: e38397.
18. Hughes, A.R. & Stachowicz, J.J. 2011. Seagrass genotypic diversity increases disturbance response via complementarity and dominance. Jour. of Ecol. 99: 445–453.
19. Espeland E.K., Emery N.C., Mercer K.L., Woolbright S.A., Kettenring K.M., Gepts P., Etterson J.R. 2017. Evolution of plant materials for ecological restoration: insights from the applied and basic literature. Jour. of App. Ecol. 54: 102–115.
20. USDA. 2006. Can Genetic Diversity be Influenced by Nursery Practices? Why We Care About Genetics Vol. 10.
21. Turesson, G. 1922. The species and the variety as ecological units. Hereditas. 3:100-13.
22. Wolfe, M. S. 1985. The current status and prospects of multiline cultivars and variety mixtures for disease control. Annu. Rev. Phytopathology. 23: 251-273.
23. Tooker, John F., and Steven D. Frank. 2012. Genotypically Diverse Cultivar Mixtures for Insect Pest Management and Increased Crop Yields. Jour. of App. Ecol. 49(5): 974–985.
24. Genung, M.A., Lessard, J.‐P., Brown, C.B., Bunn, W.A, Cregger, M.A., Reynolds, W.M.N., Felker‐Quinn, E., Stevenson, M.L., Hartley, A.S., Crutsinger, G.M., Schweitzer, J.A. & Bailey, J.K. 2010. Non‐additive effects of genotypic diversity increase floral abundance and abundance of floral visitors. PLoS ONE. 5: e8711
25. Kaut, AHEE, Mason, H.E., Navabi, A., O’Donovan, J.T., Spaner, D. 2009. Performance and stability of performance of spring wheat variety mixtures in organic and conventional management systems in western Canada. Jour. of Ag. Sci. 147: 141–153.
[EA1]Title is flexible
